Yesterday, my
mind was blown. A little chat with Steven Loiselle and I will forever
think differently about the oceans. If our eyes had better resolution and the
sun had a slightly different spatial intensity, oceans would appear purple! Loiselle
took us into the crazy world of environmental spectroscopy and gave us the
basics on solar radiation. What was supposed to be lecture on the bio-optics of
the Great African Lakes actually turned into an intriguing and perhaps novel
understanding of solar radiation, its affect on climate change, and ultimately
everything we see around us.
He starts with carbon dioxide and gave us some
info regarding green house gases and how there has been a rise in global
temperatures due to changes in the concentration of atmospheric gases. When we
think of greenhouse gases, we tend to think of CO2 as the bad guy.
Yes, it’s the primary gas responsible for green house effect enhancement but
it’s a bit more complicated than that. When the sun’s radiation hits the
planet, every entity is capable of absorbing, scattering, and transmitting
radiation; but how much arrives and how much is emitted depends on a lot of
things. Loiselle explains that how much radiation arrives is not linear with
distance but squared, and theoretically, it’s a balance between what’s going in
and what’s going out. Planck developed an equation that actually calculates spectral
distribution of a blackbody using factors like wavelength, temperature, and a
few constants. See formula below:
While ozone absorbs much of the atmospheric
radiation emitted from he sun, the rest of our ecosystem plays an equally
important role into the manifestation of energy on Earth. Most oceans, for
example, also absorb large amounts of radiation while land surfaces like
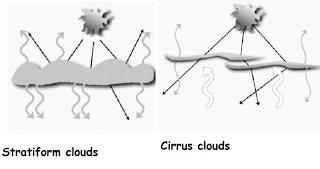
deserts, glaciers, and poles are capable of reflecting it. Clouds, which are
essentially condensed and frozen water particles, are responsible for
scattering light. How these particles come together and their conditions, give
rise to different properties that effect light scattering and how we visualize
what’s around us. Have you ever asked yourself why the sky is blue? It’s
because lights with smaller wavelengths (blues and purples) scatter more than
colors with larger wavelengths. Colors like red simple pass through the
atmosphere, and are not scattered as easily. When the sun sets, we see a
red/orange glow because the sun is at a much greater distance from the earth,
meaning there is a longer optical pathway for light. This optical pathway
allows more lights to get scattered, including colors with larger wavelengths
which would normally be difficult to scatter. If there were
no atmosphere to scatter and reflect light, then everything would be black with
the exception of the sun, which would appear white/yellow.
How water particles come together and their conditions give rise to different properties that effect light scattering and how we visualize what’s around us.
Forget the atmosphere though, Loiselle’s work
was mostly on bodies of water. He tells us there are four major things that
are responsible for absorbing and scattering incident solar radiation in water:
tripton, phytoplankton, water, and chromopheric dissolved organic matter
(CDOM). A closer look at water and we find that although the water we pour in
our glasses to drink is transparent, the water in the ocean appears blue. Why? Well,
when the sun’s white light penetrates the surface of the water and hits the
white sand at the bottom of the ocean, light scatters and we see a dark rich
blue color. In reality, this means that everything except this blue color is
being absorbed. Environmental spectroscopy, however, shows the wavelength of
light least absorbed in the ocean is purple! The oceans appears blue to us
because the our eye pigments have better resolution to blue-like colors, and they
only have the capacity to see colors of the ocean around this wavelength. This
also depends on the spectral intensity of the light source (the sun), which
does not have equal proportions of violet, blue, green and red. Putting this in
context and we can also conclude that snow is actually more blue than white because
spec analysis shows that it absorbs in red and transmits in blue.
Wavelength least absorbed in the ocean is purple
Using this fundamental understanding and
technique, we can measure how far and how much water was been polluted by
measuring the optical input of biology and chemistry in water. Loiselle looks
at water using 1) in situ
measurements and 2) temporal spatial satellites to measure what’s being
absorbed, scattered, reflected and emitted from the surface of the water. By
determining the extension of pollution in lakes, they can see how lake behavior
changes and ultimately what affect this may have on climate. Any variance in
the expected behavior of lakes and it can affect hydrology, vegetation,
biodiversity, currents, trade winds, rain patterns, ect. The list can go on and on.
Regarding modern day application, using daily
satellites has allowed Loiselle to create “modes” of behavior for lakes that
serve as a map for regions of the earth and bodies of water that are more
sensitive to climate change. If the concentration of some chemical gets too
high or the lake becomes polluted to the point where it may negatively affect the climate of surrounding area, they can work in cooperation with government
to stop water usage/flow, thereby preventing any of the aforementioned consequences. The Great African Lakes Loiselle has studied amount to about
¼ of the Earth freshwater source, and sitting idle is not worth the potential
risk. If the technology exists to monitor changes in such an influential part of the planet, we shouldn't hesitate take to advantage of it. Let’s do our part.
As a student, I have absorbed this info and am
ready to emit it back to my peers next week. My only hope is that it scatters
enough so that everyone in the class enjoys the presentation and walks away seeing
things in a different "light". After all, the ocean IS purple... right?
Sources: Steven Loiselle, his lecture, and his powerpoint (for images).





No comments:
Post a Comment