Walking through the streets of San Gimignano and seeing all
the leather stores, I wondered if it was possible to get another leather
wallet. I soon realized I didn’t need
two different types or colors of wallets (no matter how nice the Italian
leather wallets are), and we continued on our unsuccessful quest to find fedoras. Leather materials always seem so appealing to
me. They seem to last forever and can
withstand almost everything. It’s bizarre
for me to think that all the leather I’ve seen originated from animal
skin. It just doesn’t seem possible that
the two could be related. After some
research, I started to realize how complex the process is to turn animal skin
into leather - so many intricate steps, all requiring the watchful eye of a
master of the trade. Even before the
leather can be tanned, all of the flesh, fat, and hair must be removed. Then the elaborate process of tanning can
begin.
 |
| Tanneries |
Tanning is a process that permanently alters the protein
structure of the skin. It is also a
process which can provide the color for the final product. Before the tanning agents are added, the “pickling”
step increases the acidity of the hide to a pH ranging between 3.4 and 3.5, and
this range is very specific and crucial.
If the pH of the leather is too low, the leather will most likely be flat,
hard, and wet. It may also contain
grease spots on the surface. If the pH
of the final leather product is too high, the leather will probably be plump,
loose, and dry. It may also have a drawn
grain or be too soft.
After the conditions for tanning have been established, the
tanning agents can be added. Traditionally,
tanning used tannin (an acidic chemical compound) as the reagent. This is where tanning got its name. In modern times, scientists have discovered
that chromium salts are the most effective for tanning animal skin. The most common tannin agent used in chrome
tanning (tanning in which chromium salts are used) is sodium dichromate. Chromium sulfate is then produced from the
sodium dichromate. Through a series of
reactions, the chromium sulfate produces polychromium(III) compounds. These polychromium compounds are subunits
that eventually link together the collagen in the animal skin. Before the linkage occurs, other reagents are
added producing ionized carboxyl groups in the collagen. At the low pH established by the “pickling”
step, the polychromium compounds can now cross-link with the collagen by
coordinating with the ionized carboxyl groups.
The pH is slowly increased to solidify this cross-link in order to keep
the leather sturdy and durable, yet slightly malleable.
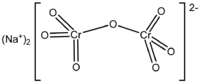 |
| Sodium Dichromate |
Once these processes are complete, the leather is then worked
and sewn into the products we buy and enjoy every day. I love both my new belt and wallet and of
course the indescribable new leather smell that always accompanies them. It’s something I can’t get anywhere
else. I used to be ignorant of how
daunting of a task it is to turn animal skin into leather, but now I can
appreciate what I’ve bought even more!
It’s amazing the science that goes into such a drastic conversion, but
its engineered has allowed leather products to be available to more and more
people.
References:
http://thephilosophyofinteriordesign.blogspot.it/2011/03/trendy-decorating-with-leather-couches.html
No comments:
Post a Comment